Flu vaccine

Flu vaccine composition selection
Flu viruses are changing constantly, by that means, the vaccine composition is reviewed every year in order to update on 3 of the following:
- Which influenza viruses are making people sick
- How efficiently can those viruses spreading
- How effective is the previous season’s vaccine protect against those viruses
Year-round surveillance for influenza will be conducted by more than 144 national influenza centers in over 114 countries. Afterward, the virus samples will be collected, tested and the laboratories will send the representative sample to WHO Collaborating Centers.
WHO will then make recommendations on the composition of the influenza vaccine. Since Northern Hemispheres and Southern Hemispheres flu season took place in different periods of time, the vaccine composition may vary between these two locations.
For Vietnam, we will have the flu vaccine composition of Northern Hemispheres, which will be available around October/November annually.
Type of flu vaccine
There are 3 main type of influenza vaccine as following:
Inactivated influenza vaccines [IIV]
This is a flu vaccine made from an inactivated influenza virus. To be precise, an influenza virus is injected into a chicken egg to replicate. Afterward, this virus is inactivated by heat, radiation, or chemicals. This inactivated virus still has the antigen which helps the immune system to create antibodies against the disease as usual.
Recombinant influenza vaccine [RIV]
This vaccine was created by recombinant technology which does not use chicken egg in the production process. This method uses protein from the virus to activate the immune system. In the laboratories, these proteins will be extracted and recombined for vaccination use.
Live attenuated influenza vaccine [LAIV]
This vaccine contains a virus that has been attenuated or weakened so that it can only cause mild signs or symptoms to no influenza, but help to activate the antibodies. According to CDC, LAIV is currently approved for use for people 2–49 years of age with no underlying medical conditions.
There are many flu vaccine options to choose from, but the most important note is everyone aged 6 months and older should get a flu vaccine every year. If you have questions regarding the flu vaccine, consult with FMP for a health care professional.
Naming Influenza Viruses
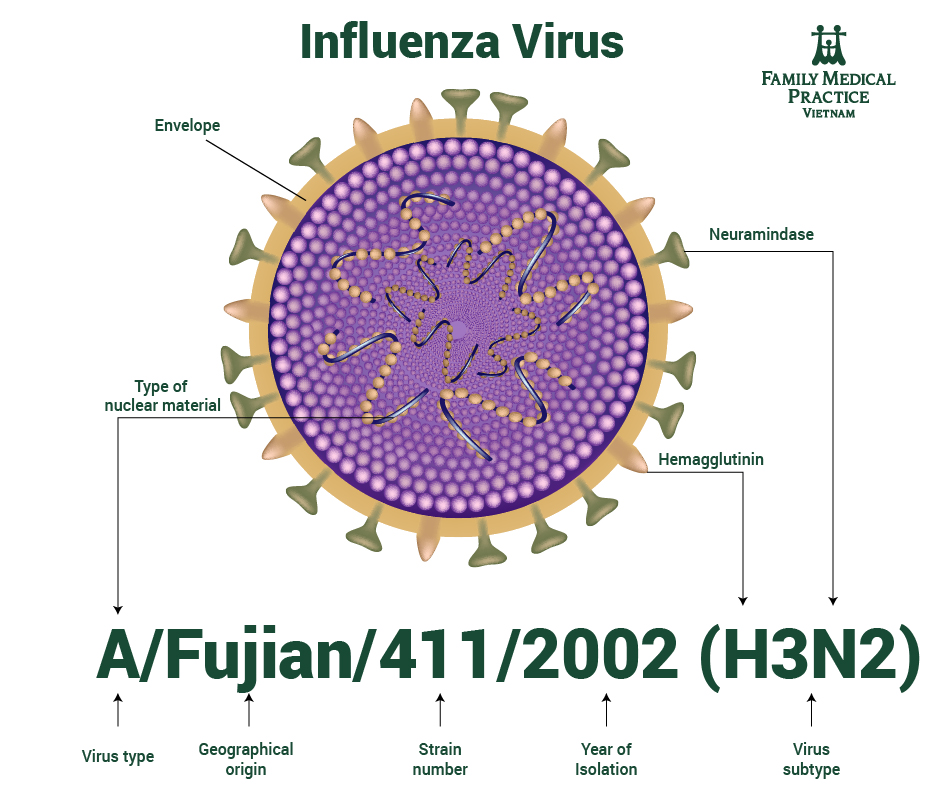
There’s a global naming convention for influenza viruses accepted by WHO as following:
- The virus type (ex., A, B, C, D)
- The host of origin (ex., duck, chicken, etc.). For human-origin viruses, no host of origin designation is given. Note the following examples:
- (Duck example): avian influenza A(H1N1), A/duck/Alberta/35/76
- (Human example): seasonal influenza A(H3N2), A/Perth/16/2019
- Geographical origin where the virus was first isolated (e.g., Washington, Phuket, etc.)
- Strain or lineage number (ex., 2570, 3073, etc.)
- Year of isolation (ex., 2009, 2020, etc.)
- For influenza A type viruses, virus protein antigen subtype described by letter and number, H1 to H16 and N1 to N9 (ex., H1N1, H3N2)
- The 2009 pandemic virus was assigned a distinct name: A(H1N1) pdm09 to distinguish it from the seasonal influenza A(H1N1) viruses that circulated prior to the pandemic.
- When humans are infected with influenza viruses that normally circulate in swine, these viruses are called variant viruses and are designated with a letter ‘v’ (ex., an A(H3N2)v virus).
Flu vaccine season 2023/2024 service at FMP Hanoi

This flu season 2022/2023, we will have the quadrivalent vaccine which can help to protect against 2 flu type A and 2 flu type B. At FMP, we will have Influvac Tetra vaccine, an inactivated influenza vaccine, with the virus strain protection as following:
A/Victoria/4897/2022 (H1N1) pdm09 - like strain
A/Darwin/9/2021 (H3N2) - like strain
B/Austria/1359417/2021 - like strain
B/Phuket/3073/2013 - like strain
Injection fee: 400.000 VND / shot
Inbox us: http://m.me/FamilyMedicalPracticeHanoi
☎️ 024 3843 0748 - 112, 119 (24/7)
Email: hanoi@vietnammedicalpractice.com
 We use cookies on this website to enhance your user experience
We use cookies on this website to enhance your user experience